Cannes Lions
ZELBORAF CAMPAIGN
VCCP HEALTH, London / ROCHE / 2014
Overview
Entries
Credits
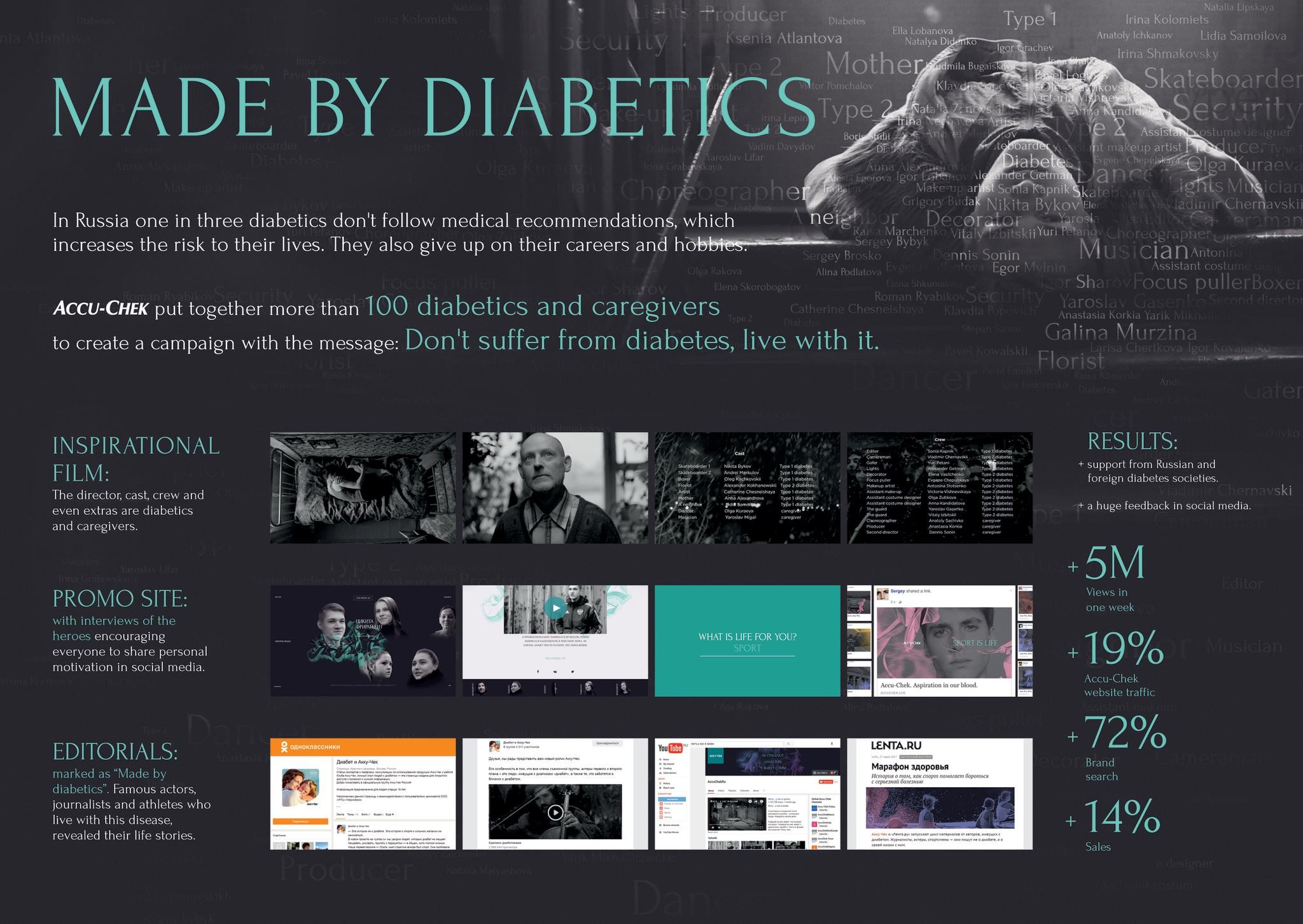
OVERVIEW
Description
Zelboraf is a treatment for skin cancer that has now spread to other parts of the body. For decades, patients with this condition have received a standard chemotherapy, which only has a 15% response rate. There is no way of knowing in advance of treatment who will be fortunate enough to respond – so everyone has to be treated in the same way. Zelboraf is different, it is the first skin cancer treatment to come with its own diagnostic tool, which is used before treatment to see who should or shouldn’t respond. In those patients positively identified with this tool there is an 85% chance of success. So with Zelboraf, patients can now be treated as individuals.
In the campaign, the mannequins represent the old, blanket approach to treatment and the single patient represents the new individual patient.
This international campaign ran all around the world, except the US and included a number of ad executions, above and below-the-line printed and digital materials, plus a patient support programme and app.
Execution
The launch campaign was used across all promotional materials (including print detail aid, electronic iPad detail aid, global website and post-launch congress activity) to create a coherent, consistent and recognisable brand.
Further materials were developed to facilitate smooth roll-out in individual countries, like a brand book, implementation guide and image library.
A global advertising schedule, targeting our key audience of specialists, ran for 6 months following launch in print and electronic media. The campaign ran for two years globally, excluding the US.
Outcome
Qualitative research with physicians showed attitudes had acutely changed.
Physicians who considered skin cancer therapy frustrating and stagnant, now felt hugely motivated. They described Zelboraf as cutting edge and unique.
By the end of 2012, in countries where Zelboraf had launched, diagnostic-testing rates exceeded 80%. This was well beyond the 2012 testing target of 60% and it continues to rise – hitting 90% by early 2013.
By the end of 2012 Zelboraf had secured an 84% global market share, surpassing the 60% target.
Total 2012 sales were 124% vs the target set by Roche.
Similar Campaigns
12 items