Pharma > Patient Engagement
OUTCARE
HAVAS HEALTH & YOU, Sao Paulo / OUTCARE / 2022

Overview
Credits
Overview
Background
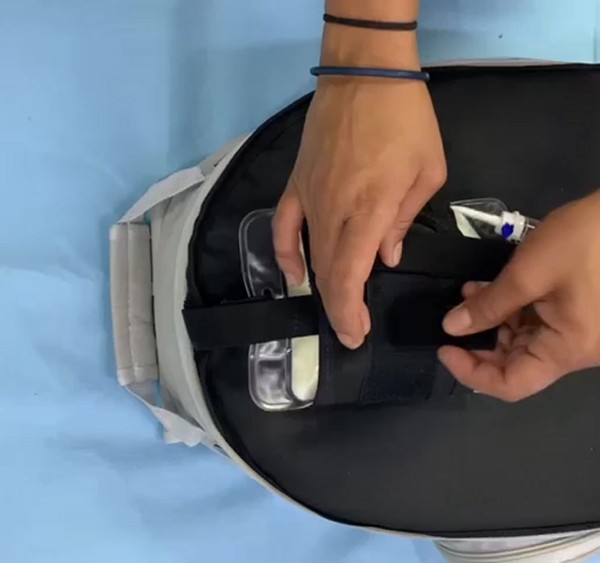
More than 290,000 electro-dependent patients are estimated to exist in Brazil. These are people who must be connected to machines 24 hours a day, seven days a week in order to survive. Many of them are children who have been sentenced to spend their entire lives within four walls. They are unable to see the sun, interact with other children, or enjoy simple childhood pleasures such as putting their feet on a sandy beach. To give these families more mobility without sacrificing safety, we created Outcare, an independent electro station with more than 8 hours of autonomy that allows them to safely store medical gadgets such as an infusion pump, gastrostomy tube, medical aspirator, total parenteral nutrition (TPN), oximeter, glucose monitor, and intravenous medication in a single volume.
Describe the creative idea
Playing in the sand, lying on the grass, or feeling the sea are all enjoyable activities.Some of the most enjoyable childhood memories are made outside. Life for electro-dependent children, on the other hand, is confined to four walls. We created Outcare to provide mobility to families with electric dependents. It is an independent station that ensures that all machines are safely stored at the same volume and have more than 8 hours of autonomy.
Our goal is that home care, an increasingly common form of de-hospitalization, does not represent a new form of imprisonment for these pediatric patients and their families, this time in their own homes. We hope that by using a portable format, we can give the children and their caregivers more mobility and freedom of access to the development opportunities available in the outside world.
Describe the strategy
Some machines are portable, but each health company is only concerned with its own market. An example is parenteral feeding backpacks. However, in practice, many patients require the use of multiple machines. Furthermore, due to a lack of usability and electricity supply, it is impossible to implement all solutions.
We had to gather a diverse range of opinions and needs in order to create Outcare, including those of doctors, nurses, engineers, experienced designers, and caregivers. A family and their doctor partnered with the product design team to develop the V1 for the station.
Describe the execution
In February 2022, we began developing V1 by gathering medical information, device safety requirements, and family insights. We are testing the first version with a family who has a child who uses five devices and gathering feedback to create three more stations that will serve patients from the Intestinal Rehabilitation Program for Patients with Ultra Short Bowel, which is overseen by Dr. Maria Fernanda Carvalho de Camargo.
Following the initial tests, we will move on to a second version of the station with technical certifications provided by Hospital Samaritano and Instituto Americas, both of which are part of UnitedHealth Group. All of Dr. Maria Fernanda's patients will benefit from this version. She will collaborate with the Americas Institute to create a clinical study on how the outdoors affects these families' quality of life.
After the study is completed, we hope to incorporate the solution into health insurance home care programs.
List the results
Outcare's immediate results are difficult to assess because it is not a traditional marketing campaign. Any technology project of this magnitude takes years to fully implement, so it will be divided into phases.
To begin, we created three outpatient models. Leo and his family use a large model that includes five devices. Two more patients are using a medium model (3 devices) and a small model (2 devices).
Based on their findings, we will manufacture more than fifty Outcare 2.0s for use in a clinical study with Hospital Samaritano and Instituto Americas to assess the quality of life of home care patients who can go outside.
We will invite investors to produce Outcare 3.0 with data, scientific foundation, and other learning, and we will try to include our autonomous station in home care programs of health insurance and the Brazilian public health service.
Describe any restrictions or regulations regarding Healthcare/RX/Pharma communications in your country/region including:
ANVISA (Brazil's equivalent to the FDA) is a health regulatory agency that prohibits the direct communication of prescription drugs to patients. We can advertise OTC products, but we can't encourage people to buy them. Hospitals provide electro-dependent machines, which are subsidized by health plans.
Describe the target audience and why your work is relevant to them.
Our aim is to supply families with pediatric patients who are currently on an exclusive home care routine and necessarily involve a combined solution that allows them to load multiple therapies and/or treatments in a single volume without the use of electrical sockets.
More Entries from HAVAS HEALTH & YOU
24 items