Innovation > Innovation
OUTCARE
HAVAS HEALTH & YOU, Sao Paulo / OUTCARE / 2022


Overview
Credits
Overview
Why is this work relevant for Innovation?
We can say, without any doubt, that Outcare transforms lives by allowing electro-dependent children not only to survive, but to live. This removes people from being confined within four walls, sharply increases the quality of life of an entire family, and includes children with chronic diseases in society. It brings together engineering, medicine and family knowledge to make it possible to have an independent station that manages to put all the devices in a single volume, with electrical autonomy, so that electro dependent children can have a childhood outdoors.
Background
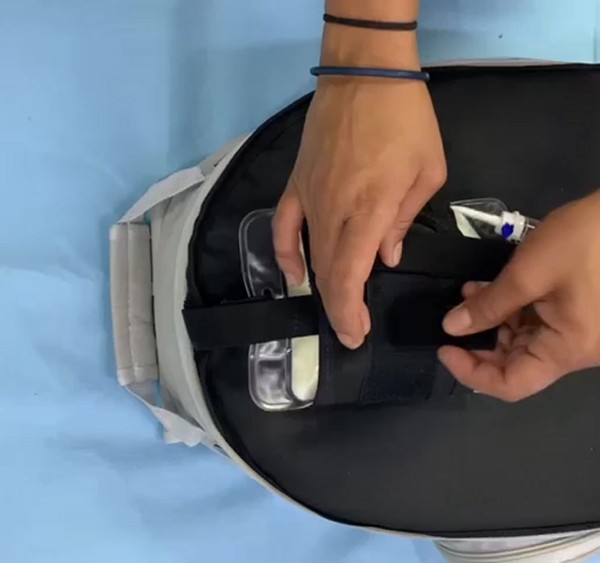
More than 290,000 electro-dependent patients are estimated to exist in Brazil. These are people who must be connected to machines 24 hours a day, seven days a week in order to survive. Many of them are children who have been sentenced to spend their entire lives within four walls. They are unable to see the sun, interact with other children, or enjoy simple childhood pleasures such as putting their feet on a sandy beach. To give these families more mobility without sacrificing safety, we created Outcare, an independent electro station with more than 8 hours of autonomy that allows them to safely store medical gadgets such as an infusion pump, gastrostomy tube, medical aspirator, total parenteral nutrition (TPN), oximeter, glucose monitor, and intravenous medication in a single volume.
Describe the idea
For electro dependent children, life is defined by four walls. Some of them will never even know a square. To provide the warmth of the sun, interaction with other children, and family outings for these children, we created Outcare: an independent station that provides mobility for electro-dependent children to have a childhood outdoors.
The compartments have been designed to store each device safely:
• Infusion bomb
• Medical Ventilator
• Medical Aspirator
• Total Parenteral Nutrition (TPN)
• oximeter
• Glucose monitor
• Intravenous medication
And the battery was chosen to provide at least 8 hours. Children assisted by Samaritano Hospital are testing Outcare's MVP to start production on a larger scale, generate clinical studies, and capture sponsorships to serve as many families as possible.
What were the key dates in the development process?
In February 2022, we began developing V1 by gathering medical information, device safety requirements, and family insights. We are testing the first version with a family who has a child who uses five devices and gathering feedback to create three more stations that will serve patients from the Intestinal Rehabilitation Program for Patients with Ultra Short Bowel, which is overseen by Dr. Maria Fernanda Carvalho de Camargo.
Following the initial tests, we will move on to a second version of the station with technical certifications provided by Hospital Samaritano and Instituto Americas, both of which are part of UnitedHealth Group. All of Dr. Maria Fernanda's patients will benefit from this version. She will collaborate with the Americas Institute to create a clinical study on how the outdoors affects these families' quality of life.
After the study is completed, we hope to incorporate the solution into health insurance home care programs.
Describe the innovation / technology
There were two main challenges in this project: packing different appliances in a single volume and having enough energy for at least 8 hours.
Each device has its correct place and fits, complying with all safety measures. Tubes cannot be bent. The battery cannot heat medication or parenteral nutrition, so it is enclosed in an insulating cover. The fan must have an airtight housing to avoid contaminating other devices. And the maximum target weight was 12kg, approximately the weight of a student's backpack.
Regarding the materials used at the station, we prioritized those that could be properly sanitized such as plastic.
As we mentioned above, we are between version 1 and 2 collecting feedback to implement the improvements. This version 2 will be tested by 36 patients from the Intestinal Rehabilitation Program for Patients with Ultra Short Boweland technicians from Instituto America will evaluate cases to issue safety certifications.
Describe the expectations / outcome
Our goal is to serve all children in home care programs through health insurance and public health services. We know that this is not an impossible goal, given that this number of patients is around 200,000. The project's scalability is linked to the number of sponsorships that we will capture after the third version. But we are also optimistic, as each unit is around US$600.
The relevance of this project is not only about the impacted families' quality of life, but about a change in treating chronic diseases that proposes a change of mindset to, instead of keeping patients alive, keeping patients living. And through Outcare we want to promote studies that prove this vision.
More Entries from Product Innovation in Innovation
24 items
More Entries from HAVAS HEALTH & YOU
24 items